Cases from the 2016 Seminar
- 1. Two Malignant Melanomas in an 18 year old
- 2. Squamous Cell Carcinoma with Perineural Involvement
- 3. Renal Cell Carcinoma Presenting as a Metastasis to Skin
- 4. Multiple Keratoacanthoma
- 5. Multinucleate Cell Angiohistiochytoma
- 6a. Desmoplastic Malignant Melanoma: A Diagnostic Pitfall
- 6b. Acral Malignant Melanoma
- 7. Recurrent Atypical Fibroxanthoma
- 8.
12. More cases
13. More cases
14. More cases
Case 1: TWO primary melanomas in an 18 year old
PRESENTERS: Charles Perniciaro, M.D., Sarah Ferrer-Bruker, D.O., Jacksonville, FL
History: An 18 year-old woman with Fitzpatrick type II skin presented for a “skin check” because she had several nevi. Two suspicious lesions were noted by the dermatologist—one on the face and one on the leg. The patient was reluctant to have a biopsy because the lesions had been checked in the past by another physician, and the patient was soon to be starting college.
Physical Examination: 6 x 8 mm irregularly pigmented and irregularly shaped brown plaque on the right cheek, and a 1 cm mm irregularly pigmented and irregularly shaped brown/black plaque on the right medial shin.
Histopathology: Cheek: irregular distribution of melanocytic nests at the dermal-epidermal junction and within the superficial dermis. Scattered melanophages and inflammation in the dermis, with features suggesting regression. No maturation of melanocytes with depth of descent into the dermis. Shin: vertically oriented nests of melanocytes at the dermal-epidermal junction and within the dermis, with intraepidermal spread of melanocytes, cytologic atypia, and epidermal “consumption” by nests of melanocytes. No maturation of melanocytes with depth of descent into the dermis.
Clinical Course: The patient was referred for surgery of both lesions. Genetic testing pending.
Diagnosis: Cheek: Primary malignant melanoma 0.25mm thickness, with regression; Shin: primary malignant melanoma 0.95mm thickness.
Points of Emphasis: Melanoma occurs in both children and young adults. An age-based retrospective study suggests the biology of pediatric and adolescent melanoma differs from conventional adult melanoma. Molecular testing and/or second opinion consultations from dermatopathologists and surgeons with wide experience in melanoma can be useful in this scenario.
References: 1. Lorimer PD, White RL, Walsh K, et al. Pediatric and Adolescent Melanoma: A National Cancer Data Base Update. Ann Surg Oncol. 2016 Jun 30. [Epub ahead of print]. 2. Wood BA. Paediatric melanoma. Pathology. 2016; 48:155-65.



CASE 2: SQUAMOUS CELL CARCINOMA WITH PERINEURAL INVOLVEMENT
PRESENTERS: Charles Perniciaro, M.D., George Schmieder, D.O., Jacksonville, FL
History: An 87 year-old man presented with a crusted lesion on the right helix. The patient had a history of several non-melanoma skin cancers in the past and was generally in excellent health. An initial shave biopsy of the lesion demonstrated a benign keratosis. Post-procedure, the man complained of considerable pain at the biopsy site, and the patient was re-evaluated one week later. A frozen section biopsy specimen revealed squamous cell carcinoma, and the patient underwent MOHS surgery. The tumor cleared in three stages. Permanent sections submitted from the MOHS procedure revealed prominent perineural squamous cell carcinoma.
Physical Examination: 1.5 x 0.8 cm crusted nodule on superior aspect of the left helix.
Histopathology: Initial shave biopsy: acanthosis with hyperkeratosis-no evidence of carcinoma; repeat biopsy with MOHS procedure: well-differentiated squamous cell carcinoma, in some areas with microscopic features suggestive of a keratoacanthoma type of SCC. Tumor was easily identified around medium-sized cutaneous nerves, a finding confirmed with a pancytokeratin (AE1/AE3) immunostain.
Clinical Course: CT imaging of the head and neck negative for evidence of metastasis of intracranial perineural carcinoma. The patient was referred for post-operative radiotherapy.
Diagnosis: Squamous cell carcinoma with prominent perineural tumor involvement.
Points of Emphasis: Sampling error or shallow shave biopsy specimens can lead to incorrect microscopic diagnosis. Squamous cell carcinomas that extend deep into the dermis or into subcutaneous tissue pose a greater risk for more aggressive biologic behavior, including metastasis. Perineural squamous cell carcinoma can extend well beyond the clinical, surgical and histopathologic margins of the tumor. Intracranial extension is possible.
References: 1. Sapir E, Tolpadi A, McHugh J et al. Skin cancer of the head and neck with gross or microscopic perineural involvement: Patterns of failure. Radiother Oncol. 2016; S0167-8140(16)31167-7. doi: 10.1016/j.radonc.2016.06.011. [Epub ahead of print]. 2. Burton KA, Ashack KA, Khachemoune A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am J Clin Dermatol. 2016 Jun 29. [Epub ahead of print]

CASE 3: Renal cell carcinoma presnting as a metastasis to to skin
PRESENTERS: Charles Perniciaro, M.D., Jessica Newburger, D.O., Karthik Krishnamurthy, D.O., Jacksonville, FL
History: A 54 year-old man, presumably in good health, presented to the dermatologist with a solitary nodule of the scalp. The clinical differential diagnosis included Merkel cell carcinoma, angiosarcoma, and pyogenic granuloma.
Physical Examination: 3.0 cm friable, erythematous to violaceous nodule on the right parietal scalp.
Histopathology: Nodular aggregate of somewhat nested cells with abundant translucent cytoplasm and indistinct cellular borders demonstrating focal areas of a highly vascular stroma. Only some of the cellular aggregates within the lesion demonstrated nuclear atypia. Tumor cells stained positive with pancytokeratin, vimentin, and the RCC stain.
Clinical Course: The patient was referred for CT imaging of the abdomen which revealed an 8.4 x 6.8 x 7.2 cm mass in the lower pole of the left kidney. A left nephrectomy was accomplished and the mass was determined to be a primary clear cell RCC of histologic grade G3-G4. The microscopic features of the primary tumor were very similar to those seen in the skin metastasis. The tumor extended into the perineal fat and the patient was staged as pT3aN0. The patient underwent adjuvant radiation therapy to the excision site of the scalp metastasis, with a total dose of 64Gy in 32 fractions. Three months later, follow-up CT scanning revealed an enlarged paratracheal lymph node, which was subsequently determined to be involved with metastatic RCC, at which time pazopanib chemotherapy was initiated.
Diagnosis: Metastatic renal cell carcinoma.
Points of Emphasis: Metastatic renal cell carcinoma to the skin is very uncommon, and is usually not clinically suspected. The “clear cell” microscopic features may simulate a benign nodular hidradenoma. The tumor is unusual in that it stains microscopically with both an epithelial marker (cytokeratin) and a mesenchymal marker (vimentin).
References: 1. Weiss L, Harlos JP, Torhorst J, et al.. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605-12. 2. Smyth LG, Casey RG, Quinlan DM. Renal cell carcinoma presenting as an ominous metachronous scalp metastasis. Can Urol Assoc J. 2010;4:E64-6.





CASE 4: Multiple Keratoacanthomas
PRESENTERS: Jordan Brooks, MD, Jacquelyn Swietlik, MD, Andrea Murina, MD New Orleans, LA
History: A 57 year old male with a history of NMSC presented to our clinic for evaluation of multiple, new “skin cancers” on his bilateral forearms and hands. The patient noted that he had had multiple surgeries, skin grafts, and radiation in the past 20 years for “too many to count” skin cancers on upper extremities. He previously followed closely with a plastic surgeon and dermatologist at an outside hospital but could not continue care after losing health insurance. In the interim, he had noticed multiple new growths over the left hand and bilateral forearms that were rapidly growing in size. All lesions were painful and bled intermittently similarly to previous skin cancers. He was hopeful that they would go away on their own, as a few had in the past. His newest lesion was one week old at presentation and had grown rapidly since its first appearance. The lesion on the left hand was the oldest and largest and was very concerning to the patient because he works as a plumber. Past Medical History: Diabetes Mellitus Type II, Hypertension. No history of melanoma. Past Surgical History: Multiple excisions and skin grafts to bilateral forearms, radiation. Medications: Metformin, Lisinopril, Statin, baby aspirin Allergies: No known allergies. Family History: No family history of similar lesions, no family history of melanoma. Social History: Positive for tobacco use: 1 pack per day. Patient denies any alcohol or illicit drug use.
Physical Examination: General: Well appearing, well-nourished Caucasian male in no apparent distress. Skin: Right upper extremity: Hypopigmented plaques/scars over areas of prior skin grafts. Erythematous papule with central hemorrhagic crust over extensor upper arm, hyperkeratotic 8 mm erythematous papule with central punctum over proximal anterior wrist, and a similar erythematous papule with central punctum and hemorrhagic crust over the distal forearm. Left upper extremity: Hypopigmented patches/ scars over areas of previous skin grafts. 4.5 X 3.5 cm plaque with central punctum and hemorrhagic crust over his left dorsal hand and a similar 2.5 X 1.5 cm plaque with a central punctum and hemorrhagic crust over the left lateral forearm. Face: Actinic damage, two large, soft, fatty subcutaneous tumors right nasal bridge and left jawline. No oral lesions.
Histopathology: Left lateral arm: Squamous cell carcinoma, keratoacanthoma type with positive margins to the base of the specimen; Right lateral wrist: Squamous cell carincoma, keartoacanthoma type with free margins; Right extensor arm: Squamous cell carcinoma, invasive, well-differentiated with free margins.
Clinical Course: Initially, we excised three of the patient’s smaller lesions. For the large lesion over the left hand, the patient has been referred for radiation therapy. Patient did have recurrence of left hand lesion and continued to develop new lesions. He was treated with serial injections of 5-FU intralesional. The patient has since been lost to follow up.
Diagnosis: Multiple Keratoacanthomas
Points of Emphasis: Keratoacanthomas (KAs) often present as single, rapidly growing, dome shaped nodules with a central keratin plug1. Normally developing spontaneously, KA’s have been reported to develop following trauma, administration of certain medications (RAF and BRAF- inhibitors), and in association with underlying malignancy. KA’s usually present as solitary lesions between 5-15 mm in size. However, there are many other clinical presentations of KA’s. Examples include giant KA’s, keratoacanthoma centrifugum marginatum, intraoral KA’s, multiple spontaneously regressing KA’s (aka Ferguson-Smith syndrome), and generalized eruptive KA’s of Grzybowski. Ferguson- Smith syndrome is a rare condition that follows an autosomal dominant inheritance pattern and often presents in adolescence with multiple KA’s over sun-exposed areas. These lesions usually resolve in weeks to months. Generalized eruptive KA’s of Grzybowski typically presents in middle-aged adults with hundreds of small KA’s. These lesions often lead to a masked facies in a periorbital distribution, known as the sign of Zorro.
KA’s typically progress through three stages of development that differ clinically and histologically: the early growing phase, stationary phase, and senescent phase. On histology, KA’s appear “volcano-like” during the first two stages; they are comprised of “epithelial lips”, or well-differentiated keratinocytes, surrounding a keratin-filled crater. There is often a surrounding inflammatory response composed of lymphocytes and eosinophils. Neutrophils may also be present in small abscesses within the tumor. During the senescent phase, fibrosis begins at the base and the lesions flatten.
Our case illustrates the presentation of multiple spontaneous KA’s. Our patient has no family history of KA’s and his lesions are larger than would be expected in Ferguson-Smith syndrome. He reports regression of some lesions in the past, which would be expected in Ferguson-Smith syndrome. However, some were persistent and required aggressive therapy. His clinical picture does not fit with generalized eruptive KA’s of Grzybowski as his lesions are larger in size and fewer in number than would be expected in this condition. He is not immunocompromised and is not on any medications associated with development of KA’s. The patient’s smoking history could be a potential contributing factor to his increased risk of developing KA’s. An animal study with mice showed an association between direct skin exposure to tobacco extracts and the development of KA’s. A more recent case-control study also found an association between cigarette smoking and the development of solitary KA’s. In this study, smoking was more prevalent in patients with solitary KA’s than in controls, with an odds ratio (OR) of 9.16.
Treatment options noted in the literature for multiple KA’s include excision, oral retinoids, intralesional fluorouracil, and oral erlotinib1. Upon review of the literature, one case report of a 27-year-old woman with diabetes mellitus type II and multiple spontaneous KA’s thought to be secondary to nonfamilial Ferguson-Smith-type KA’s, reports successful lesion regression following oral and topical retinoid therapy. Our patient is a treatment challenge because he presented with multiple spontaneous KA’s including one large, rapidly proliferating KA over his left hand. Treatment options for large, rapidly proliferating KA’s include radiation therapy, intralesional 5-fluorouracil, intralesional methotrexate, intralesional methotrexate followed by surgery, and intralesional interferon. Close follow-up is important, as there have been reports of reactive KA’s following different treatment modalities. Counseling regarding tobacco use is also important in management of our patient in order to eliminate all underlying risk factors.
References: 1. Owen C, et al. Treatment Of Skin Disease. 4th ed. Elsevier; 2014:358-360. 2. Zhu L, Likhari S, Stebbins W. A Case of Multiple Large Reactive Keratoacanthomas Treated With Serial Zinc Oxide Wraps. Dermatologic Surgery. 2015;41(6):750-753. 3. Bolognia J et al. Dermatology. [Philadelphia]: Elsevier Saunders; 2012.




CASE 5: Multinucleate Cell angiohistiocytoma
PRESENTER: James S. Petit, BS, Laura C. Cleary, MD, Daniel J. Sheehan, MD, Loretta S. Davis, MD, Augusta, GA
History: A 10 year-old boy with no personal or family history of skin disease presented with a growth on his left index finger which had been present for several months. Since first appearing, the growth had been slowly enlarging and bled easily with minimal trauma.
Physical Examination: Physical examination revealed a bright red, friable 5 mm papule on the volar surface of the left index finger
Histopathology: Histology showed a dermal vascular proliferation with an edematous fibrotic stroma and numerous scattered multinucleate cells. Immunohistochemical staining of the multinucleate cells was positive for CD68 and negative for CD34.
Clinical Course: A shave removal was performed. The patient and his parents were reassured of the benign nature of this diagnosis and that no further treatment was necessary.
Diagnosis: Multinucleate cell angiohistiocytoma (MCAH)
Points of Emphasis: MCAH is a rare and benign skin condition first described in 1985 by Smith and Wilson Jones.1 Currently, about 150 cases have been documented. MCAH manifests as a red to violaceous papule, or as multiple papules, and most commonly involves the lower extremities and hands. However, other locations such as the forehead and cheeks have been reported.1,2 MCAH typically appears insidiously over the course of weeks to months and does not regress spontaneously.2The vast majority of patients are asymptomatic but common symptoms include pruritus and bleeding. Histologic findings of MCAH include a dermal proliferation of superficial capillaries and venules with scattered multinucleate cells containing angulated cytoplasm; occasional lymphocytes and other inflammatory cells may be present.3,4 With immunohistochemistry, the multinucleate cells stain positive for CD68 and negative for CD34. MCAH is a rare and likely under diagnosed benign vascular proliferation. Until recently, this neoplasm was reported to occur exclusively in the adult population.
References: 1. Danielle S. Applebaum, Fareesa Shuja, Lindsey Hicks et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatology Online Journal 20 (5): 4. 2. Pérez LP, Zulaica A, Rodríguez L, et al. Multinucleate cell angiohistiocytoma. Report of five cases. J Cutan Pathol. 2006 May; 33(5):349-52. 3. Nikita Lakdawala, Michael Murphy, Justin Finch. A pediatric case of multinucleate cell angiohistiocytoma responsive to intralesional triamcinolone. Journal of the American Academy of Dermatology , Volume 74 , Issue 5 , AB40




CASE 6a: Desmoplastic Malignant Melanoma: A diagnostic pitfall
PRESENTERS: Michael James Davis, BMus; Dipti Anand, MD, Atlanta, GA
History: A 66-year-old Caucasian woman presented for her routine physical examination and was found to have a lesion on her left upper arm. Physical examination and review of systems were otherwise negative.
Physical Examination: A 0.5 x 1.0 cm poorly defined, asymmetric pink plaque with irregular borders and subtle dotted vessels was present on the left upper arm. A shave biopsy with the clinical impression of basal cell carcinoma vs squamous cell carcinoma was performed.
Histopathology: Histology showed dermal scarring with stromal perivascular lymphocytic inflammation, and slight junctional melanocytic proliferation. S-100, Melan-A and SOX-10 immunohistochemical markers highlighted the patchy atypical junctional melanocytic proliferation, with S-100 and SOX-10 also showing focal and weak staining of the dermal spindle cells. Additional information revealed no prior history of biopsy at this site. The pathology was signed out as “atypical compound melanocytic proliferation with scar”, with recommendation for complete removal of the lesion and correlation with the residuum in the re-excision specimen.
A full thickness re-excision of the site showed, in association with scar of the previous procedure, classic appearing melanoma in-situ with lentiginous, contiguous junctional melanocytic proliferation of single and nests of enlarged, atypical melanocytes. In the dermis was a diffuse proliferation of cytologically banal, delicate spindle cells with mildly enlarged and slightly hyperchromatic nuclei and occasional dermal mitoses (~2/mm2). Associated trailing lymphoid aggregates were noted in the stroma. S-100 and SOX-10 diffusely highlighted the dermal melanocytic proliferation which was negative for Melan-A.
Diagnosis: Invasive Malignant Melanoma, Desmoplastic type, with a Breslow thickness of 2.1 mm, Clark’s level 4, and with a pathologic tumor stage of pT3a.
Points of Emphasis: Desmoplastic Melanoma (DM) is a rare cutaneous malignancy that proves a challenge for both clinicians and pathologists because of its subtle presentation and broad differential diagnosis. It is typically found on chronically sun-damaged skin of older individuals and has a male to female ratio of approximately 2:1. DM often presents as an amelanotic plaque or an ill-defined scar-like lesion that lacks the varied pigmentation, typically seen in conventional melanomas. Its most common location is head and neck region, arising in association with lentigo maligna melanoma. Compared to conventional melanoma, DM has a lower rate of nodal metastasis despite greater median tumor thickness at presentation. It however, presents with an increased risk of distant metastasis to visceral sites, such as the lung, and has a relatively higher incidence of local recurrence.
Histologically, DM remains a challenging diagnosis and necessitates a high index of suspicion. Atypical spindle cells are found in a densely hyalinized collagenous stroma. There can be deep infiltration and invasion into the subcutis, and DM has a high propensity for perineural invasion. Trailing lymphoid aggregates can be a subtle clue in reaching the diagnosis. An important pitfall to appreciate is that DM can be negative for melanocytic markers including Melan-A, Mart-1, and HMB-45. In this case, the tumor was negative for Melan-A.
In summary, both pathologists and dermatologists need to be aware of this vaiant of melanoma. Minimal pigmentation & scar-like presentation of the disease can be misleading, both clinically & histologically, resulting in delayed or mis diagnosis.Diagnosis is dependent upon an awareness of the condition & often requires the inclusion of a battery of immunohistochemical markers to establish the histogenesis of the tumor infiltrate
References: 1. Han, D et al. “Clinicopathologic Predictors of Survival in Patients with Desmoplastic Melanoma.” PLoS One. 2015;10:e0119716. 2. Chen LL, BA, Jaimes, N, Barker CA, Busam KJ, Marghoob, AA. “Desmoplastic melanoma: A review.” J Am Acad Dermatol. 2013;68:825-833.




CASE 6b: Acral malignant melanoma
PRESENTERS: Karla Paz, MD, Juana Garza-Chapa, MD, Candelario Rodriguez, MD, Jorge Ocampo-Candiani, MD, Monterrey, México
History: 54 year old Hispanic man presented to our clinic with a 4-year history of a hyperpigmented lesion on the sole of his left foot that had been previously treated as an infected necrotic ulcer with oral antibiotics and one episode of surgical debridement during the past year. The patient referred purulent secretion, spontaneous bleeding and associated pain at the site of the lesion along with 6kg weight loss during the last year. He denied any previous trauma to the site.
Physical Examination: Irregular brown-black colored plaque with an eccentrically located tumor with ulceration and a macular component showing multiple colors: light brown, dark brown and black. The lesion was located on the distal plantar side of the left foot affecting the 4th and 5th toe. Dermoscopý showed a parallel-ridge pattern at the borders of the lesion, irregular diffuse pigmentation with blue-white veil. Lymphadenopathy was absent.
Laboratory Data: Chest and abdomen CT scan failed to demonstrate lymphadenopathy and metastatic disease.
Histopathology: Histological sections showed hyperkeratosis, acanthosis and lentiginous proliferation of atypical melanocytes along the base and sides of rete ridges with vertical growth phase extending to the dermis, Breslow’s depth of 4.87mm, Clark level IV, mitotic rate of 25 mitosis/mm2, with ulceration and perineural invasion. Regression, lymphovascular invasion and inflammatory infiltrate were absent.
Clinical Course: Amputation of the left foot was done followed by inguinal lymph node resection. A total of 6 lymph nodes were resected, but unfortunately 1of them was positive for melanoma cells. Because clinical stage was IIIC (T4N3Mx), chemotherapy or radiotherapy was indicated.
Diagnosis: Acral malignant melanoma
Points of Emphasis: Acral melanoma (AM) is defined as melanoma affecting the palms, soles and nail apparatus and it is the most prevalent type of malignant melanoma in non-Caucasian populations. On histology, it presents a diffuse proliferation of large atypical melanocytes along the epidermal-dermal junction which is dispersed in a lentiginous pattern with marked acanthosis and elongation of the rete ridges. Contrary to what has been suggested, sun exposure is a significant risk factor for ALM and incidental foot trauma leads patient attention to the lesion rather than being a risk factor. ALM is associated with poorer prognosis than other types of melanoma because its diagnosis is usually delayed compared to other cutaneous melanomas, translating into a thicker and deeper lesion by time of recognition. Delay in diagnosis can be due to multiple factors including relative inaccessibility for self-examination, particularly for melanomas located on the feet, in addition to the many common dermatological conditions that can appear on the feet and lead to misdiagnosis, such as fungal infections, warts, hematoma, nevi, paronychia and chronic wounds mainly related to diabetic ulcers, peripheral occlusive arterial disease, and posttraumatic ulcers. When evaluating chronic wound located on the foot, it should be considered into account that a complete re-epithelialization of feet wounds may take longer than 8 weeks. However, there should be a healing tendency under adequate therapy; if that tendency is not observed a biopsy should be taken to rule out malignancy.
In summary, acral melanomas, especially those located on the feet, represent a diagnostic pitfall, as they usually have a delayed diagnosis or tend to be misdiagnosed. Greater awareness of this entity and earlier biopsies of suspicious feet lesions should be encouraged.
References: 1. Bristow I R & Acland K. (2008) Acral lentiginous melanoma of the foot and ankle: A case series and review of the literature. Journal of Foot and Ankle Research 1:11 2. Curtin JA, et.al. (2005) Distinct sets of genetic alterations in melanoma. N. Engl J Med 353: 2135-2147

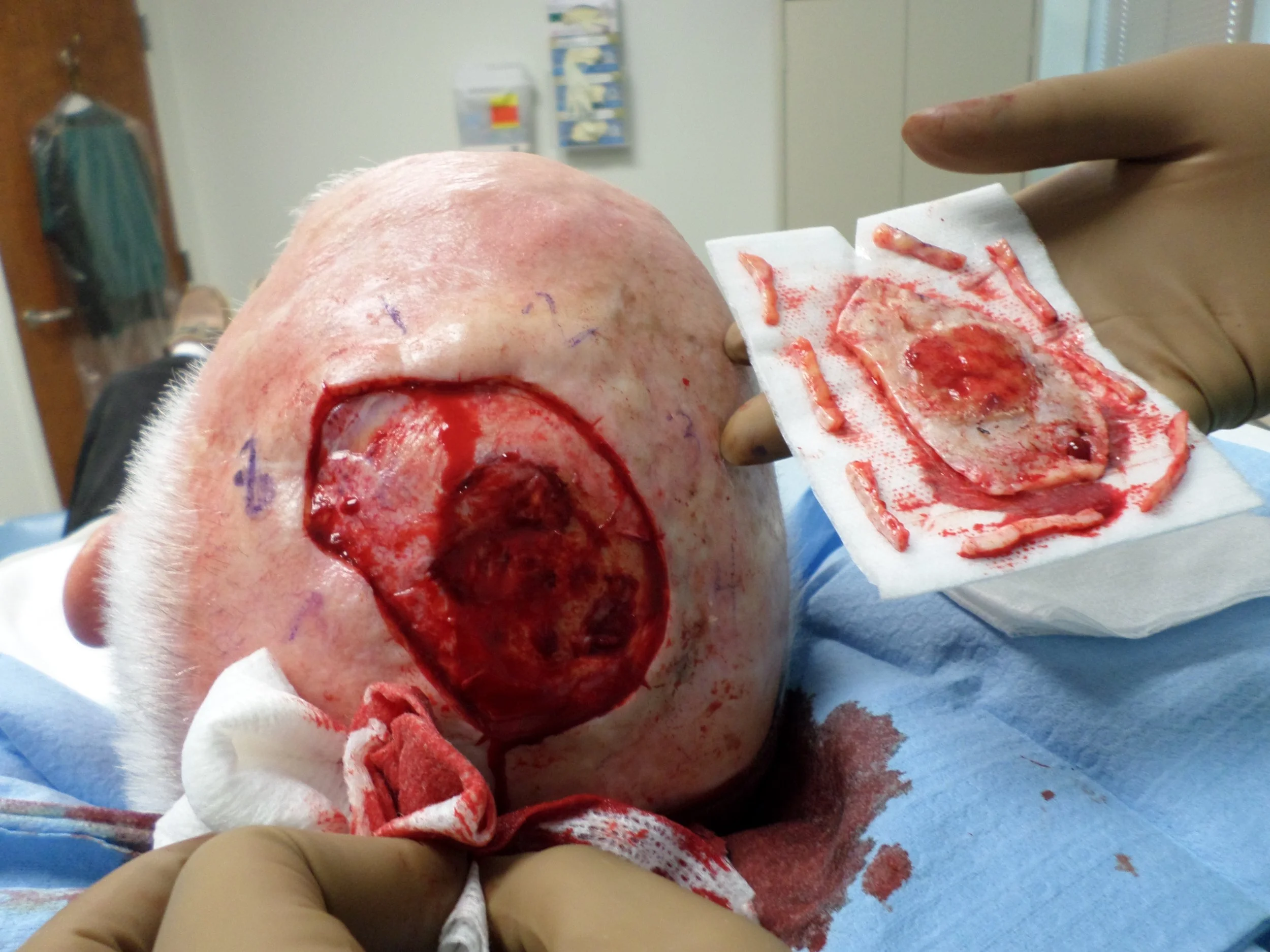
CASE 7: Recurrent atypical fibroxanthoma
PRESENTERS: Elizabeth Hargroder, MS-IV; Aimee Coscarart, MD; Diane Trieu, MD; Michael Lee, MD; New Orleans, LA
History: 77 yo male presented with a bleeding lesion on his left mid-parietal scalp. His past medical history is significant for squamous cell carcinoma of the scalp, status post excision and radiation in 2007. He subsequently developed a new bleeding lesion within the radiation zone in 2013. Biopsy showed atypical fibroxanthoma (AFX) and the patient was referred to Mohs surgery. Positive deep margins to calvarium necessitated plastic surgery referral for bone drilling and closure with full thickness skin graft. Patient reported the wound never completely healed despite routine wound care and hyperbaric oxygen therapy. Rebiopsy of the lesion on 7/11/16 was consistent with recurrent AFX.
Physical Examination: 8 x 5cm erythematous friable tumor on vertex of the scalp
Histopathology: Skin with dome-shaped lesion characterized by collection of spindle, oval, and round mesenchymal cells in the dermis displaying atypical nuclei, with multinucleation and foamy cytoplasm, and with numerous mitotic figures. The tumor cells are highlighted on CD68 and CD10 stains, negative AE1/AE3, CD31, and desmin. S100 shows focal positive cells.
Clinical Course: 1 stage of Mohs micrographic surgery was performed to obtain peripheral surgical margins free of tumor. Deep margins remained positive with evidence of tumor invading down to the calvarium. 14 tissue blocks were processed for frozen sections until clear margins were detected, resulting in a surgical defect 8.2 x 6cm.
Diagnosis: Recurrent Atypical Fibroxanthoma
Points of Emphasis: AFX is an uncommon rapidly growing cutaneous neoplasm of uncertain etiology. It commonly occurs on photo-damaged skin on the scalp of an elderly man, clinically presenting as a nodular or polypoid tumor accompanied by ulceration or bleeding. These tumors behave indolently, despite their highly anaplastic cytology. In a retrospective chart review of nearly 3,000 cases of AFX, the twenty-year disease specific survival remained 97.8% [3]. 7.6% of patients experienced local recurrence after clear margin excision and 2.75% later developed metastasis, with no significant difference in rates following wide local excision verses Mohs microsurgery [3]. Although these more aggressive AFXs are rare, some argue that malignant fibrous histiocytoma should be considered in the context of recurrence or metastasis [1]. Similar to our case presented above, Leeet al presented an elderly individual with a history of therapeutic radiation exposure who developed AFX within the exposed area, underwent Mohs surgery, and later experience AFX recurrence at the initial site [4].
Histologically, AFX presents as a well-circumscribed tumor of fasicularly arranged pleomorphic epitheloid, spindled, and multinucleated giant cells within and confined to the dermis [5]. Frequent atypical mitoses are often seen, requiring negative immunostaining for S100, cytokeratin, CD34, and desmin as a perquisite to diagnosis to rule out other poorly differentiated malignant neoplasms [5]. Although no immunohistochemical markers are specific for AFX, immunostaining is required for diagnosis. Case reviews of AFXs show staining to be positive for A1AT, CH10, CD68, CD74, CD99, melanoma-associated antigen (NK1/C3), vimentin, SMA, cathepsin B, p53 and stromelysin-3 in greater than 90% of cases, while 90% stain negative for cytokeratins, S100, desmin, CD15, NGFR, and human melanoma black 45 (HMB-45) [3]. However, cell morphology and immunohistochemistry alone is not sufficient to exclude the more aggressive pleomorphic dermal sarcoma (PDS). These tumors are indistinguishable from AFX by single cell analysis but are asymmetrical and poorly circumscribed, with or without infiltration of surrounding subcutaneous tissue, nerves, and lymphatic vessels [5]. Although debate exists, some conclude that AFX represents the non-infiltrating precursor lesion of PDS [2].
References: 1. Branch, LG, Albertini, JG, & Leshin, B. (2015). Recurrent Atypical Fibroxanthoma verses malignant fibrous histiocytoma. Journal of Craniofacial Surgery, 26 (4). Dio.10.1097/scs.0000000000001550. 2. Helbig, D, Ihle, MA, Putz, K, Tantcheva-Poor, I, Mauch, C, Buttner, R, & Quaas, A. (2016). Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget, 7 (16), 21763-21774.